Deciphering the proximal TCR signaling network in mice and humans
T lymphocytes are essential cells of the immune system, that specifically recognize a wide range of pathogens, but also malignant cancer cells in the body. Upon binding to specific antigens, the T-cell receptor (TCR) triggers a signaling cascade encoded by the rapid and successive phosphorylation of many intracellular proteins. This small chemical modification promotes their conformational change, their association with other partners, and the activation of protein kinases that in turn also phosphorylate other protein targets. This ultimately promotes the activation of T‐cells, which undergo morphological changes, synthetize new proteins, and secrete cytokines.
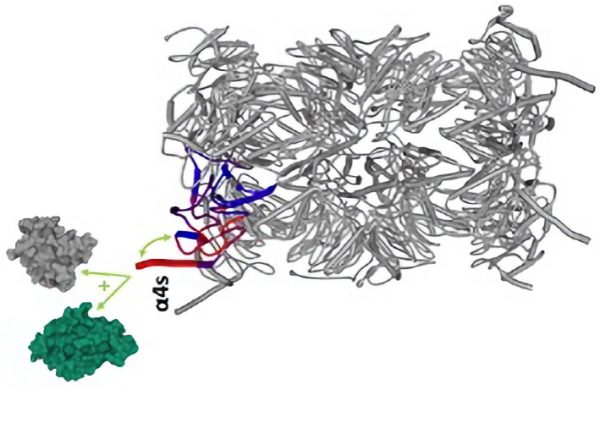
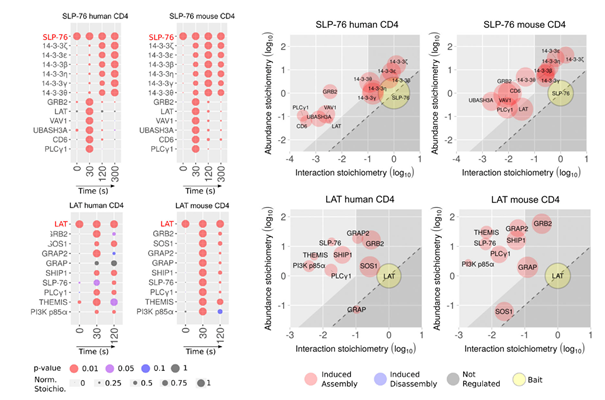
Recent advances in mass spectrometry and bioinformatics analysis now allow a better characterization of the signalling pathways based on protein phosphorylation. Using high-throughput phosphoproteomic methods, we could previously monitor the phosphorylation kinetics of around 7000 phosphosites during the first 10min following TCR engagement in primary T lymphocytes 1. Another approach is to purify the protein complexes formed upon TCR stimulation, and to analyse their composition by mass spectrometry 2,3. In collaboration with immunologists from the group of B. Malissen (CIML, Marseille), we recently implemented this interactomics approach in human T-cells4, using gene-edited primary lymphocytes in which several key proteins were tagged to allow affinity purification of the signalosome assembling upon TCR engagement. Our study revealed a high degree of conservation of the TCR-signaling network between human and mouse T-cells. Using an inhibitor of the LCK tyrosine kinase, we illustrate how this approach can be used to determine the mechanism of action of drugs targeting human T cell activation.
(1) Locard-Paulet, M.; Voisinne, G.; Froment, C.; Menoita, M. G.; Ounoughene, Y.; Girard, L.; Gregoire, C.; Mori, D.; Martinez, M.; Luche, H.; Garin, J.; Malissen, M.; Burlet-Schiltz, O.; Malissen, B.; Gonzalez de Peredo, A.; Roncagalli, R. LymphoAtlas: A Dynamic and Integrated Phosphoproteomic Resource of TCR Signaling in Primary T Cells Reveals ITSN 2 as a Regulator of Effector Functions. Molecular Systems Biology 2020, 16 (7). https://doi.org/10.15252/msb.20209524.
(2) Voisinne, G.; Kersse, K.; Chaoui, K.; Lu, L.; Chaix, J.; Zhang, L.; Goncalves Menoita, M.; Girard, L.; Ounoughene, Y.; Wang, H.; Burlet-Schiltz, O.; Luche, H.; Fiore, F.; Malissen, M.; Gonzalez de Peredo, A.; Liang, Y.; Roncagalli, R.; Malissen, B. Quantitative Interactomics in Primary T Cells Unveils TCR Signal Diversification Extent and Dynamics. Nat Immunol 2019, 20 (11), 1530–1541. https://doi.org/10.1038/s41590-019-0489-8.
(3) Mori, D.; Grégoire, C.; Voisinne, G.; Celis-Gutierrez, J.; Aussel, R.; Girard, L.; Camus, M.; Marcellin, M.; Argenty, J.; Burlet-Schiltz, O.; Fiore, F.; Gonzalez de Peredo, A.; Malissen, M.; Roncagalli, R.; Malissen, B. The T Cell CD6 Receptor Operates a Multitask Signalosome with Opposite Functions in T Cell Activation. J Exp Med 2021, 218 (2), e20201011. https://doi.org/10.1084/jem.20201011.
(4) Nicolas, P.; Ollier, J.; Mori, D.; Voisinne, G.; Celis-Gutierrez, J.; Gregoire, C.; Perroteau, J.; Vivien, R.; Camus, M.; Burlet-Schiltz, O.; Gonzalez de Peredo, A.; Clémenceau, B.; Roncagalli, R.; Vié, H.; Malissen, B. Systems-Level Conservation of the Proximal TCR Signaling Network of Mice and Humans. J Exp Med 2022, 219 (2), e20211295. https://doi.org/10.1084/jem.20211295.