Understanding how T lymphocytes discriminate between antigens of varying affinities
T lymphocytes are essential cells of the immune system, and have the ability to sense a wide range of antigenic peptides bound to the Major histocompatibility complex (MHC). But they trigger a proper response only after recognition of high-affinity antigens coming from pathogens, while interaction with self-peptides fail to activate them. Through an in-depth, time-resolved phosphoproteomic and interactomic analysis of the signalling events taking place in T cells upon stimulation with peptides of various affinity, scientists at Proteotoul and CIML have studied the mechanisms that govern this very specific discrimination. This work was published on Sept 23,2022 in Nature Immunology.
The activation, differentiation and phenotypic response of T lymphocytes depend on the specificity and strength of the signals delivered by the T cell receptor (TCR) upon recognition of an antigenic peptide. Although low-affinity self-peptides derived from host proteins can bind the TCR, they do not trigger a response, even if present at high concentration. Conversely, even small quantities of foreign antigenic peptide that bind to the TCR with high affinity, are sufficient to fully activate a T cell. To achieve such a specificity, it has been postulated that the TCR uses a kinetic proofreading mechanism, based on the presence of multiple biochemical intermediate steps between antigen binding and a final irreversible step leading to full activation. This introduces a delay that enables only high-affinity antigens, that remain bound to the TCR long enough, to complete the series of signalling steps leading to T cell activation.
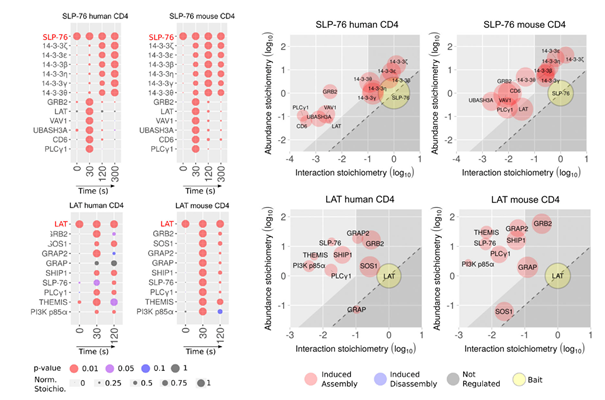
TCR stimulation promotes the rapid and successive phosphorylation of many intracellular proteins, along with with protein-protein interactions and formation of complexes. To characterize in more details these mechanisms, and identify the key steps involved in antigen discrimination, scientists analysed in a global manner all the molecular events taking place in T cells when the TCR is engaged using peptide ligands with decreasing affinities. They applied mass spectrometry-based approaches to monitor the phosphorylation kinetics of several thousand proteins, and the formation of complexes around different important molecules of the pathway.
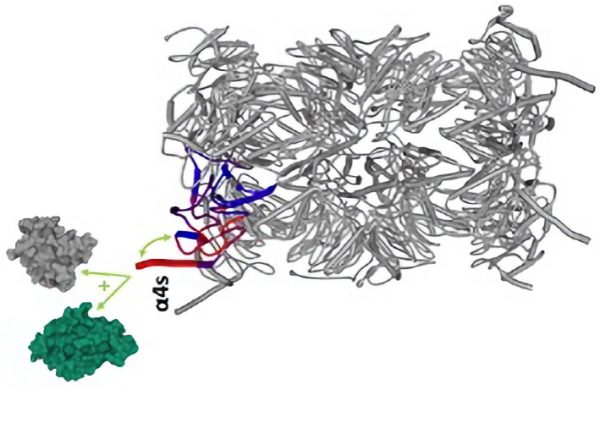
By doing so, they classified in unbiased way the events that were independent of ligand affinity, those showing a response scaling with ligand affinity, and those elicited only by strong ligands. Some upstream molecular events appeared to be totally unaffected by the differences in ligand affinities, for example the phosphorylation of the CD3 chains of the TCR, and the recruitment of the kinase ZAP70 to the TCR. Differences between strong ligands and weak ligands were rather reflected by changes in the final step of trans-autophosphorylation of ZAP70 catalytic domain, required for full activation of the kinase, and in the formation of the LAT signalosome, that was severely impaired with weak ligands. The assembly of the CD6 signalosome was only partially affected, and it still contained inhibitory molecules that might play a role in dampening TCR signals in conditions of stimulation with low-affinity ligands.
This study provides fundamental knowledge about how the series of signalling steps downstream of the TCR plays a role in antigen discrimination, in accordance with the kinetic proofreading model. Such insights into the mechanisms of TCR signalling might be useful in the future for the rational design of T cell chimeric receptors used for anti-cancer immunotherapy. It also provides a useful data resource for researchers, pointing at new candidate molecules that might play a role in the TCR pathway.

The T cell receptor signaling is triggered when the TCR binds to an antigenic peptide bound to MHC, leading to phosphorylation of CD3 chains and recruitment of the ZAP70 kinase. (a) Stimulation with high-affinity ligands allows to complete all the steps of ZAP70 phosphorylation, leading to full activation of the kinase and formation of the LAT signalosome, orchestrating the T cell response. (b) Alternatively, when the TCR binds to a lower-affinity peptide presented by MHC, the half-life of the interaction is not long enough to permit step 3 of ZAP70 activation to take place. Stimulation with weak peptides only allows the formation of the CD6 signalosome, which is able to drive a negative feedback loop.
Référence
Voisinne, G., Locard-Paulet, M., Froment, C., Maturin, E., Menoita, M.G., Girard, L., Mellado, V., Burlet-Schiltz, O., Malissen, B*., Gonzalez de Peredo, A*., Roncagalli, R. *, 2022. Kinetic proofreading through the multi-step activation of the ZAP70 kinase underlies early T cell ligand discrimination. Nat Immunol.
https://doi.org/10.1038/s41590-022-01288-x
https://hal.archives-ouvertes.fr/hal-03767648
Contacts
Chercheur l Anne Gonzalez de Peredo l T 05 61 17 55 41 l gonzalez@ipbs.fr