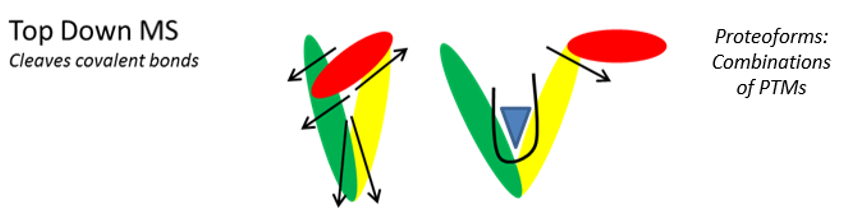
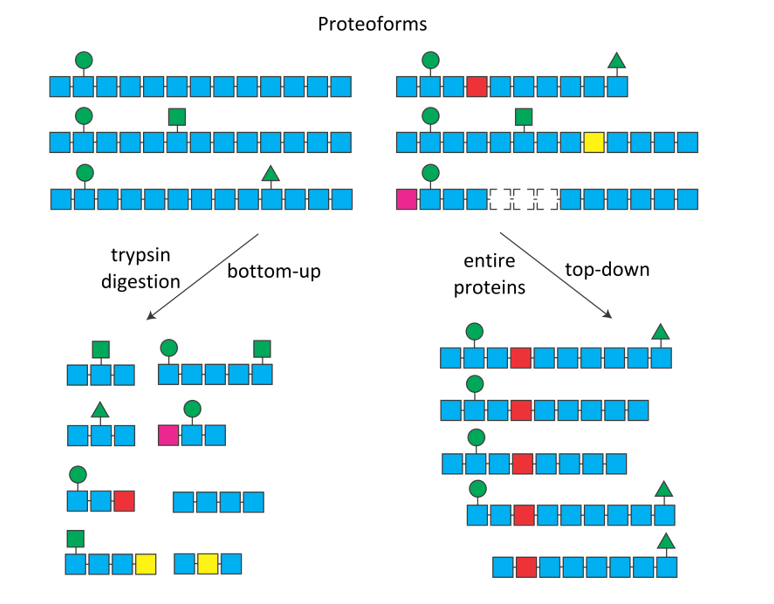
 Figure 1. The combination of post-translational modifications (green symbols), mutations (colored squares) and alternative splicing (dotted squares), can sometimes produce numerous protein isoforms of proteoforms. After digestion (bottom-up, left), it is impossible to know the origin of the trypsic peptides. In top-down (right), each proteoform is selected and fragmented individually.
Figure 1. The combination of post-translational modifications (green symbols), mutations (colored squares) and alternative splicing (dotted squares), can sometimes produce numerous protein isoforms of proteoforms. After digestion (bottom-up, left), it is impossible to know the origin of the trypsic peptides. In top-down (right), each proteoform is selected and fragmented individually.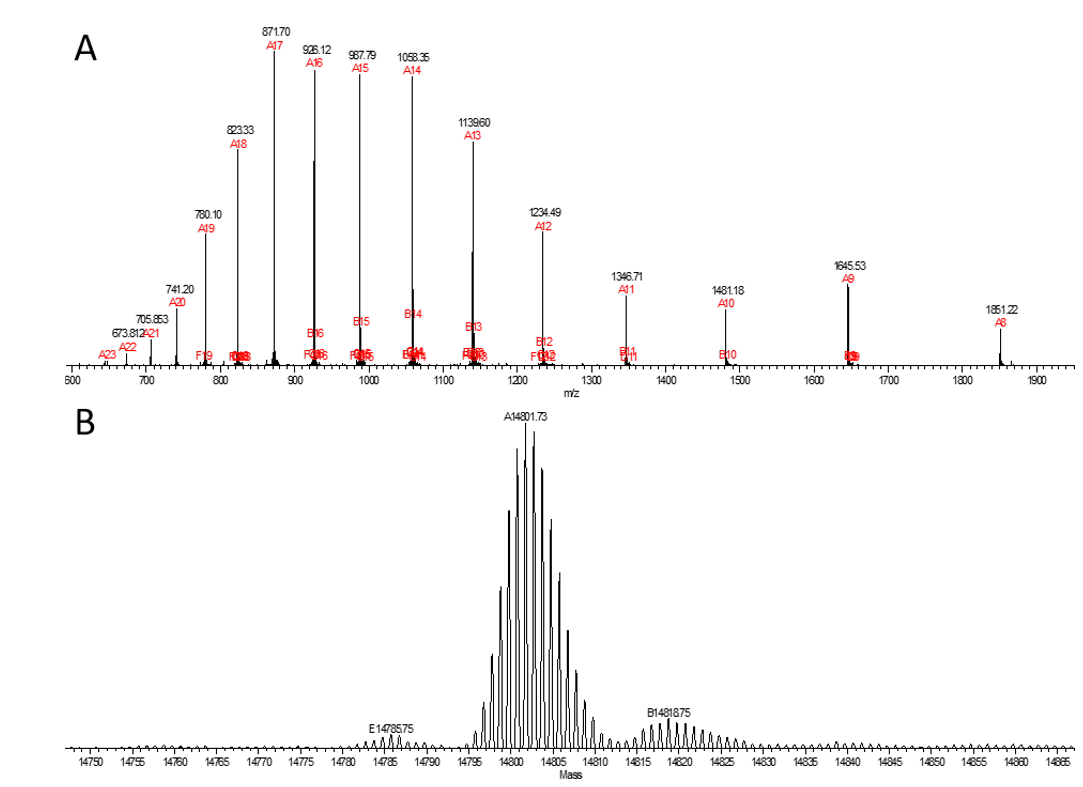
Figure 2. MS spectra corresponding to a ~15 kDa protein.
For more complex mixtures, raw data can be automatically deconvoluted and 3D plots generated, showing the pattern of the most abundant proteins (Figure 3). Targeted top-down analysis can provide information on post-translational modifications (PTMs) and protein identification.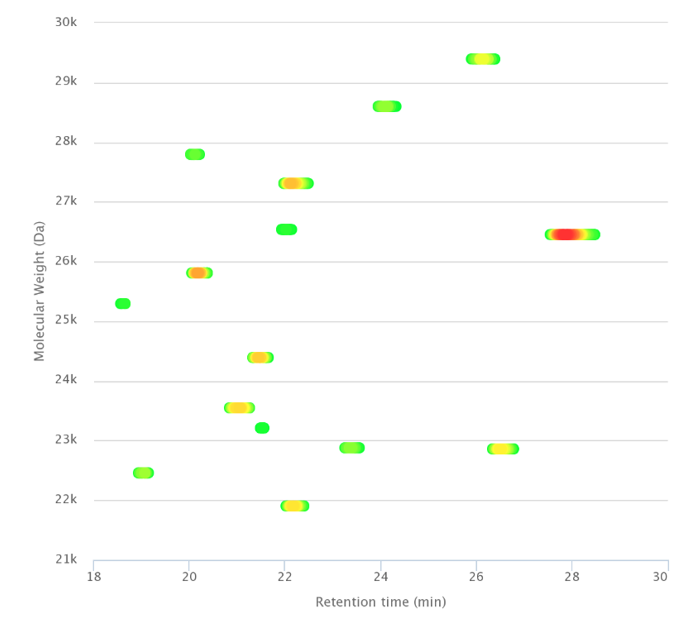
Figure 3. Separation of entire proteins after immunopurification.
2020
Lesne J, Locard-Paulet M, Parra J, Zivković D, Menneteau T, Bousquet MP, Burlet-Schiltz O, Marcoux J. Conformational maps of human 20S proteasomes reveal PA28- and immuno-dependant inter-ring crosstalks. Nature Communications 11(1):6140.
2019
Lesne J, Bousquet M-P, Marcoux J and Locard-Paulet M. Top-down and intact protein MS data visualization for proteoform analysis using VisioProt-MS. Bioinformatics and Biology Insights 13:1177932219868223.
2018
Gervais V, Muller I, Mari PO, Mourcet A, Movellan K, Ramos P, Marcoux J, Guillet V, Javaid S, Burlet-Schiltz O, Czaplicki G, Milon A, Giglia-Mari G. Small molecule-based targeting of TTD-A dimerization to control TFIIH transcriptional activity represents a potential strategy for anticancer therapy. Journal of Biological Chemistry 293(39):14974-14988.
Locard-Paulet M, Parra J, Albigot R, Mouton-Barbosa E, Bardi L, Burlet-Schiltz O, Marcoux J. VisioProt-MS: interactive 2D maps from intact protein mass spectrometry. BioInformatics 35(4):679-681.
2017
Parra J, Marcoux J, Poncin I, Canaan S, Herrmann JL, Nigou J, Burlet-Schiltz O, Rivière M. Scrutiny of Mycobacterium tuberculosis 19kDa antigen proteoforms provides new insights in the lipoglycoprotein biogenesis paradigm. Scientific Reports 7:43682.
Carel C, Marcoux J, Parra J, Réat V, Latgé G, Laval F, Burlet-Schiltz O, Demange P, Milon A, Daffé M, Tropis M, Renault M. Post-translational O-mycoloylation mediates protein targeting to the mycomembrane in C. glutamicum. Proceedings of the National Academy of Sciences 114(16) : 4231-4236.
